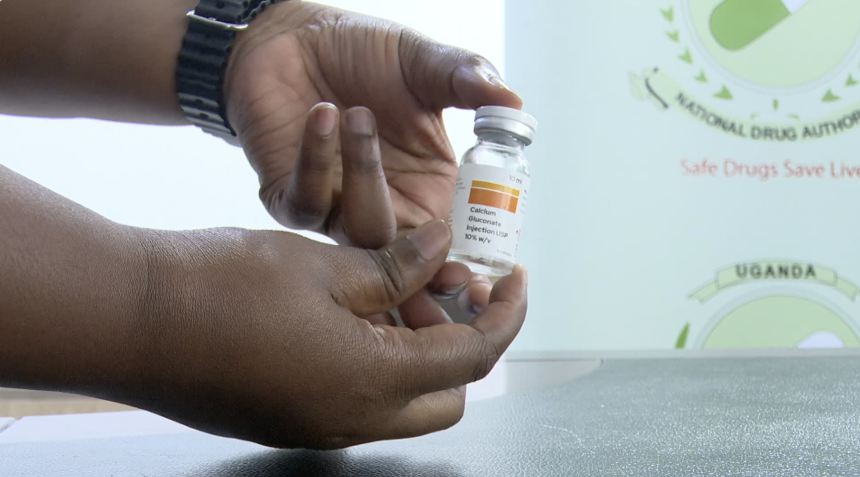
The National Drug Authority (NDA) has recalled a batch of Calcium Gluconate injection—commonly used in managing heart conditions and calcium deficiencies—after it was found to contain visible particles in a cloudy solution, rendering it unfit for use.
The contamination was discovered during routine surveillance in health facilities. According to the NDA, more than 12,000 ampoules had already been distributed to various health facilities by the National Medical Stores.
Health workers have been advised to immediately stop using the affected batch and return it for safe disposal.